Русский | English
Oncology: |
| Melanoma |
Pathogenetic treatment of primary and metastatic melanoma with disulfiram, CuSO4 and ZnSO4 |
|
Share this: Read this article in PDF format Fine antitumoral effects of these three drugs against melanoma in vitro and in vivo were presented in articles [9-18].
Also, for intratumoral treatment may be used the liquid form of disulfiram (disulfiram long - ampoules). This algorithm of the pathogenetic treatment may help to decrease mortality and may help to increase a survival of patients with melanoma. Probably it will help to raise the 5 years survival rate up to 50 % in the patients with secondary metastatic melanoma.
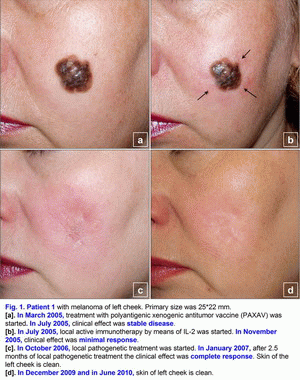
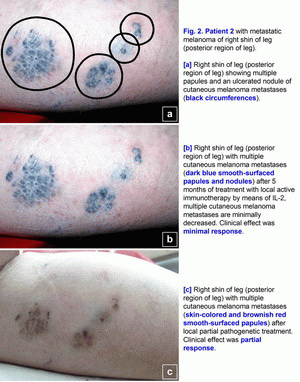
Clinical cases of the pathogenetic treatment of patients with primary and metastatic melanomaCase 1.A 42- year-old woman presented in March 2005 with melanoma (T4(?)N0M0; Breslow thickness, 4-5 mm) on the left cheek (Fig. 1a). Primary size was 25*22 mm, estimated area of excision is 45*43 mm. Melanoma was confirmed cytologically. No lymph node or visceral metastases were found after staging procedures (x-ray of the thorax and sonography of the abdomen and the regional lymph nodes) were performed [1-6]. This localization of large melanoma has a high risk of local and distant relapse (recurrences) after operation. In March 2005, treatment with polyantigenic xenogenic antitumor vaccine (PAXAV) was started (Fig. 1a). In July 2005, clinical effect was stable disease [7, 8]. In July 2005, local active immunotherapy by means of IL-2 was started. In November 2005, clinical effect was minimal response (Fig. 1b). In November 2005, treatment with polyantigenic xenogenic antitumor vaccine (PAXAV) plus BCG was started [7]. In October 2006, clinical effect was minimal response (Fig. 1b). In October 2006, local pathogenetic treatment (disulfiram plus CuSO4 - drops) was started [9-18]. In January 2007, after 2.5 months of local pathogenetic treatment (disulfiram plus CuSO4 - drops) the clinical effect was complete response (Fig. 1c). In December 2009 and in June 2010, skin of left cheek is clean (Fig. 1d). Treatment of primary melanoma was without surgery, chemotherapy and X-ray therapy. Treatment of this patient demonstrates the possibility of local treatment of large primary melanoma (disulfiram plus CuSO4 - drops) in problem localizations [9-18]. If there is a possibility of local treatment of large primary melanoma in problem localizations, hence there is a possibility of local treatment in other localizations without surgery, chemotherapy and X-ray therapy. The presumable results of treatment efficacy (complete regressions) may be: 100% for size < 1.0 cm, 95% for size from 1.0 to 2.0 cm, 80-90% for size > 2.0 cm. Risk for local recurrence of melanoma head/neck is 9.4 (table No. 3 - Balch C.M. et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann. Surg. Oncology. 2001; 8(2):101-108 [2]). In this case there is the possibility to create new forms of drugs. Case 2.A 63-year-old woman presented in August 2001 with a malignant melanoma (T3N0M0; Clark level IV; Breslow thickness, 5-6 mm) on her right shin of leg (posterior region of leg), which was initially treated with wide excision [1-6]. In June 2003, several skin lesions of metastatic melanoma were noted on the right shin of leg (posterior region of leg). Multiple new skin-colored, brownish red, and dark blue smooth-surfaced and eroded papules and nodules of metastatic melanoma appeared on the whole right lower leg in June 2003 (Fig. 2a). There was an unresectable form of secondary metastatic melanoma. A large ulcerated nodule (1.8 cm in diameter) was noted on the right posterior crural region (Fig. 2a). Metastatic melanoma was confirmed cytologically. No lymph node or visceral metastases were found after staging procedures (x-ray of the thorax and sonography of the abdomen and the regional lymph nodes) were performed.In June 2003, treatment with polyantigenic xenogenic antitumor vaccine (PAXAV) was started [7, 8]. In October 2003, clinical effect was stable disease. In October 2003, local active immunotherapy by means of IL-2 was started and was finished in February 2004. Clinical effect was minimal response (Fig. 2b). In February 2004, local partial pathogenetic treatment (zinc ointment) and PAXAV plus IL-2 were started. In January 2006, clinical effect was partial response (Fig. 2c). No lymph node or visceral metastases were found after staging procedures (x-ray of the thorax and sonography of the abdomen and the regional lymph nodes) were performed. Treatment of metastatic melanoma was without surgery, chemotherapy and X-ray therapy. Treatment of this patient demonstrates the possibility of use of local partial pathogenetic treatment (zinc ointment) in patients with secondary metastatic melanoma (multiple metastases in skin).
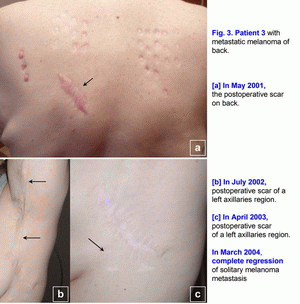
Case 3.A 42-year-old woman presented in May 2001 with melanoma (T2N0M0; Clark level III) on her back, which was initially treated with wide excision (Fig. 3a). [1-6] In July 2002, local melanoma metastases were noted in lymph nodes of a left axillaries region (N3), which was treated with wide excision (Fig. 3b). Metastatic melanoma was confirmed histopathologically. It was a first relapse. In March 2003, regional nodal metastases of melanoma were noted in the postoperative scar of a left axillaries region (N3), which was treated with wide excision (Fig. 3c). Metastatic melanoma was confirmed histopathologically. It was a second relapse. In May 2003, treatment with polyantigenic xenogenic antitumor vaccine (PAXAV) was started [7, 8]. In October 2003, clinical effect was progression of disease. Multiple (innumerable) metastases (6-36 mm) of melanoma were noted in lymph nodes of a left axillaries region and solitary melanoma metastasis (40*17 mm) was noted in the postoperative scar (N3). It was a third relapse. In October 2003, treatment with mixed vaccine (PAXAV plus BCG) and local active immunotherapy by means of IL-2 were started [7]. In January 2004, clinical effect was minimal response. In March 2004, complete regression of solitary melanoma metastasis was noted in the postoperative scar after local mixed vaccine (PAXAV plus BCG) therapy (Fig. 3c). From March 2004 to April 2005, partial regression of multiple metastases of melanoma (decrease of sizes and quantity of metastases) were noted in lymph nodes of a left axillaries region. From March 2005 to January 2006, a decrease of metastases quantity of multiple metastases of melanoma (7 lymph nodes) was noted in lymph nodes of a left axillaries region. Clinical effect was minimal response. In April 2006, multiple distant hypoechogenic lymph nodes (neck, region of pancreas, inguinal lymph node - multiple melanoma metastases M1a - ?) were noted during usual mixed vaccine (PAXAV plus BCG) and interferon alpha therapy. It was a fourth relapse. In April 2006, treatment with mixed vaccine (PAXAV plus BCG) was stopped and another therapy was started. In October 2006, clinical effect was complete (inguinal lymph node) and partial (neck, region of pancreas) regression of multiple melanoma metastases M1a (?). In October 2006, pathogenetic treatment (disulfiram plus ZnSO4 - tablets) was started [9-18]. In April 2007, clinical effect was complete regression of hypoechogenic lymph nodes in region of pancreas and neck. From April 2007 to August 2010, complete regression of multiple melanoma metastases (3-6 mm) was noted in lymph nodes of a left axillaries region. Unfortunately, this patient has only a very slow regression, but she lives. Treatment of metastatic melanoma was without surgery, chemotherapy and X-ray therapy. Treatment of this patient demonstrates the possibility of use of systemic pathogenetic treatment of patients with secondary metastatic melanoma (multiple metastases in lymph nodes). The presumable results of treatment efficacy (complete and partial regressions) may be 50-70%. But there is the possibility of local and systemic pathogenetic treatment of patients with a primary melanoma T1-4N0-3M0, stage III [9-18]. Also, there is the possibility of intratumoral pathogenetic treatment (liquid form of disulfiram [disulfiram long] (ampoules) plus ZnSO4 - tablets) of patients with subcutaneous metastases or intrahepatic metastases. This algorithm of the pathogenetic treatment may help to decrease mortality and may help to increase a survival of patients with melanoma. Probably it will help to raise the 5 years survival rate up to 50 % in the patients with secondary metastatic melanoma (table No. 1-2 - Balch C.M. et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1-4 mm melanomas. Ann. Surg. Oncology. 2001; 8(2):101-108) and Balch C.M. et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin 2004; 54(3):131-149 [2, 4]). This algorithm of pathogenetic treatment of primary and metastatic melanoma with disulfiram (drops, tablets, sterile tablets (Esperal), ampoules), CuSO4 (drops) and ZnSO4 (zinc ointment, tablets) may be very simple and very effective. References (Staging System for Cutaneous Melanoma):
References (Pathogenetic treatment of melanoma with disulfiram):
|
|
|